It would be useful to start with a definition of the concept of alkanes. These are saturated or saturated. We can also say that these are carbons in which the connection of C atoms is carried out through simple bonds. The general formula is: CnH₂n+ 2.
It is known that the ratio of the number of H and C atoms in their molecules is maximum when compared with other classes. Due to the fact that all valences are occupied by either C or H, the chemical properties of alkanes are not clearly expressed, so their second name is the phrase saturated or saturated hydrocarbons.
There is also an older name that best reflects their relative chemical inertness - paraffins, which means “devoid of affinity.”
So, the topic of our conversation today is: “Alkanes: homological series, nomenclature, structure, isomerism.” Data regarding their physical properties will also be presented.
Alkanes: structure, nomenclature
In them, the C atoms are in a state called sp3 hybridization. In this regard, the alkane molecule can be demonstrated as a set of tetrahedral C structures that are connected not only to each other, but also to H.
Between the C and H atoms there are strong, very low-polar s-bonds. Atoms always rotate around simple bonds, which is why alkane molecules take on various shapes, and the bond length and the angle between them are constant values. Shapes that transform into each other due to the rotation of the molecule around σ bonds are usually called conformations.

In the process of abstraction of an H atom from the molecule in question, 1-valent species called hydrocarbon radicals are formed. They appear as a result of not only but also inorganic compounds. If you subtract 2 hydrogen atoms from a saturated hydrocarbon molecule, you get 2-valent radicals.
Thus, the nomenclature of alkanes can be:
- radial (old version);
- substitution (international, systematic). It was proposed by IUPAC.
Features of radial nomenclature
In the first case, the nomenclature of alkanes is characterized as follows:
- Consideration of hydrocarbons as derivatives of methane, in which 1 or several H atoms are replaced by radicals.
- High degree of convenience in the case of not very complex connections.
Features of substitution nomenclature
The substitutive nomenclature of alkanes has the following features:
- The basis for the name is 1 carbon chain, while the remaining molecular fragments are considered as substituents.
- If there are several identical radicals, the number is indicated before their name (strictly in words), and the radical numbers are separated by commas.
Chemistry: nomenclature of alkanes
For convenience, the information is presented in table form.
Substance name | The basis of the name (root) | Molecular formula | Name of carbon substituent | Carbon Substituent Formula |
The above nomenclature of alkanes includes names that have developed historically (the first 4 members of the series of saturated hydrocarbons).
The names of unexpanded alkanes with 5 or more C atoms are derived from Greek numerals that reflect the given number of C atoms. Thus, the suffix -an indicates that the substance is from a series of saturated compounds.

When composing the names of unfolded alkanes, the main chain is the one that contains the maximum number of C atoms. It is numbered so that the substituents have the lowest number. In the case of two or more chains of the same length, the main one becomes the one that contains the largest number of substituents.
Isomerism of alkanes
The parent hydrocarbon of their series is methane CH₄. With each subsequent representative of the methane series, a difference from the previous one is observed in the methylene group - CH₂. This pattern can be traced throughout the entire series of alkanes.
The German scientist Schiel put forward a proposal to call this series homological. Translated from Greek it means “similar, similar.”
Thus, a homologous series is a set of related organic compounds that have the same structure and similar chemical properties. Homologues are members of a given series. Homologous difference is a methylene group in which 2 neighboring homologues differ.
As mentioned earlier, the composition of any saturated hydrocarbon can be expressed using the general formula CnH₂n + 2. Thus, the next member of the homologous series after methane is ethane - C₂H₆. To convert its structure from methane, it is necessary to replace 1 H atom with CH₃ (figure below).

The structure of each subsequent homolog can be deduced from the previous one in the same way. As a result, propane is formed from ethane - C₃H₈.
What are isomers?
These are substances that have an identical qualitative and quantitative molecular composition (identical molecular formula), but a different chemical structure, and also have different chemical properties.
The hydrocarbons discussed above differ in such a parameter as boiling point: -0.5° - butane, -10° - isobutane. This type of isomerism is called carbon skeleton isomerism; it belongs to the structural type.
The number of structural isomers increases rapidly as the number of carbon atoms increases. Thus, C₁₀H₂₂ will correspond to 75 isomers (not including spatial ones), and for C₁₅H₃₂ 4347 isomers are already known, for C₂₀H₄₂ - 366,319.
So, it has already become clear what alkanes are, homologous series, isomerism, nomenclature. Now it’s worth moving on to the rules for compiling names according to IUPAC.

IUPAC nomenclature: rules for the formation of names
First, it is necessary to find in the hydrocarbon structure the carbon chain that is longest and contains the maximum number of substituents. Then you need to number the C atoms of the chain, starting from the end to which the substituent is closest.
Secondly, the base is the name of an unbranched saturated hydrocarbon, which, in terms of the number of C atoms, corresponds to the main chain.
Thirdly, before the base it is necessary to indicate the numbers of the locants near which the substituents are located. The names of the substituents are written after them with a hyphen.
Fourthly, in the case of the presence of identical substituents at different C atoms, the locants are combined, and a multiplying prefix appears before the name: di - for two identical substituents, three - for three, tetra - four, penta - for five, etc. Numbers must be separated from each other by a comma, and from words by a hyphen.
If the same C atom contains two substituents at once, the locant is also written twice.
According to these rules, the international nomenclature of alkanes is formed.

Newman projections
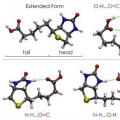
This American scientist proposed special projection formulas for graphical demonstration of conformations - Newman projections. They correspond to forms A and B and are presented in the figure below.

In the first case, this is an A-occluded conformation, and in the second, it is a B-inhibited conformation. In position A, the H atoms are located at a minimum distance from each other. This form corresponds to the highest energy value, due to the fact that the repulsion between them is greatest. This is an energetically unfavorable state, as a result of which the molecule tends to leave it and move to a more stable position B. Here the H atoms are as far apart as possible from each other. Thus, the energy difference between these positions is 12 kJ/mol, due to which the free rotation around the axis in the ethane molecule, which connects the methyl groups, is uneven. After entering an energetically favorable position, the molecule lingers there, in other words, “slows down.” That is why it is called inhibited. The result is that 10 thousand ethane molecules are in the inhibited form of conformation at room temperature. Only one has a different shape - obscured.
Obtaining saturated hydrocarbons
From the article it has already become known that these are alkanes (their structure and nomenclature were described in detail earlier). It would be useful to consider ways to obtain them. They are released from natural sources such as oil, natural, and coal. Synthetic methods are also used. For example, H₂ 2H₂:
- Hydrogenation process CnH₂n (alkenes)→ CnH₂n+2 (alkanes)← CnH₂n-2 (alkynes).
- From a mixture of C and H monoxide - synthesis gas: nCO+(2n+1)H₂→ CnH₂n+2+nH₂O.
- From carboxylic acids (their salts): electrolysis at the anode, at the cathode:
- Kolbe electrolysis: 2RCOONa+2H₂O→R-R+2CO₂+H₂+2NaOH;
- Dumas reaction (alloy with alkali): CH₃COONa+NaOH (t)→CH₄+Na₂CO₃.
- Oil cracking: CnH₂n+2 (450-700°)→ CmH₂m+2+ Cn-mH₂(n-m).
- Gasification of fuel (solid): C+2H₂→CH₄.
- Synthesis of complex alkanes (halogen derivatives) that have fewer C atoms: 2CH₃Cl (chloromethane) +2Na →CH₃- CH₃ (ethane) +2NaCl.
- Decomposition of methanides (metal carbides) by water: Al₄C₃+12H₂O→4Al(OH₃)↓+3CH₄.
Physical properties of saturated hydrocarbons
For convenience, the data is grouped into a table.
Formula | Alkane | Melting point in °C | Boiling point in °C | Density, g/ml |
0.415 at t = -165°С |
||||
0.561 at t= -100°C |
||||
0.583 at t = -45°C |
||||
0.579 at t =0°C |
||||
2-Methylpropane | 0.557 at t = -25°C |
|||
2,2-Dimethylpropane | ||||
2-Methylbutane | ||||
2-Methylpentane | ||||
2,2,3,3-Tetra-methylbutane | ||||
2,2,4-Trimethylpentane | ||||
n-C₁₀H₂₂ | ||||
n-C₁₁H₂₄ | n-Undecane | |||
n-C₁₂H₂₆ | n-Dodecane | |||
n-C₁₃H₂₈ | n-Tridecan | |||
n-C₁₄H₃₀ | n-Tetradecane | |||
n-C₁₅H₃₂ | n-Pentadecan | |||
n-C₁₆H₃₄ | n-Hexadecane | |||
n-C₂₀H₄₂ | n-Eicosane | |||
n-C₃₀H₆₂ | n-Triacontan | 1 mmHg st | ||
n-C₄₀H₈₂ | n-Tetracontane | 3 mmHg Art. | ||
n-C₅₀H₁₀₂ | n-Pentacontan | 15 mmHg Art. | ||
n-C₆₀H₁₂₂ | n-Hexacontane | |||
n-C₇₀H₁₄₂ | n-Heptacontane | |||
n-C₁₀₀H₂₀₂ |
Conclusion
The article examined such a concept as alkanes (structure, nomenclature, isomerism, homologous series, etc.). A little is said about the features of radial and substitutive nomenclatures. Methods for obtaining alkanes are described.
In addition, the article lists in detail the entire nomenclature of alkanes (the test can help you assimilate the information received).
The table shows some representatives of a number of alkanes and their radicals.
|
Formula |
Name |
Radical name |
|||||||||||
|
CH3 methyl |
|||||||||||||
|
C3H7 cut |
|||||||||||||
|
C4H9 butyl |
|||||||||||||
|
isobutane |
isobutyl |
||||||||||||
|
isopentane |
isopentyl |
||||||||||||
|
neopentane |
neopentyl |
||||||||||||
|
The table shows that these hydrocarbons differ from each other in the number of groups - CH2 -. Such a series of similar structures, having similar chemical properties and differing from each other in the number of these groups is called a homologous series. And the substances that make it up are called homologues. Homologs - substances similar in structure and properties, but differing in composition by one or more homologous differences (- CH2 -)
Carbon chain - zigzag (if n ≥ 3) σ - bonds (free rotation around bonds) length (-C-C-) 0.154 nm binding energy (-C-C-) 348 kJ/mol All carbon atoms in alkane molecules are in a state of sp3 hybridization
the angle between the C-C bonds is 109°28", therefore the molecules of normal alkanes with a large number of carbon atoms have a zigzag structure (zigzag). The length of the C-C bond in saturated hydrocarbons is 0.154 nm (1 nm = 1 * 10-9 m). a) electronic and structural formulas; b) spatial structure
4. Isomerism- STRUCTURAL isomerism of the chain with C4 is characteristic One of these isomers ( n-butane) contains an unbranched carbon chain, and the other, isobutane, contains a branched one (isostructure). The carbon atoms in a branched chain differ in the type of connection with other carbon atoms. Thus, a carbon atom bonded to only one other carbon atom is called primary, with two other carbon atoms - secondary, with three - tertiary, with four - quaternary. With an increase in the number of carbon atoms in the molecules, the possibilities for chain branching increase, i.e. the number of isomers increases with the number of carbon atoms. Comparative characteristics of homologues and isomers
1. They have their own nomenclature radicals(hydrocarbon radicals)
| |||||||||||||
From a chemical point of view, alkanes are hydrocarbons, that is, the general formula of alkanes includes exclusively carbon and hydrogen atoms. In addition to the fact that these compounds do not contain any functional groups, they are formed only through single bonds. Such hydrocarbons are called saturated.
Types of Alkanes
All alkanes can be divided into two large groups:
- Aliphatic compounds. Their structure has the form of a linear chain, the general formula of aliphatic alkanes is C n H 2n+2, where n is the number of carbon atoms in the chain.
- Cycloalkanes. These compounds have a cyclic structure, which causes their chemical properties to differ significantly from linear compounds. In particular, the structural formula of alkanes of this type makes their properties similar to alkynes, that is, hydrocarbons with a triple bond between carbon atoms.
Electronic structure of aliphatic compounds
This group of alkanes can have either a linear or branched hydrocarbon chain. Their chemical activity is low compared to other organic compounds, since all bonds within the molecule are saturated.
The molecular formula of aliphatic alkanes indicates that their chemical bond has sp 3 hybridization. This means that all four covalent bonds around the carbon atom are absolutely equal in their characteristics (geometric and energetic). With this type of hybridization, the electron shells of the s and p levels of carbon atoms have the same elongated dumbbell shape.
Between the carbon atoms the bond in the chain is covalent, and between the carbon and hydrogen atoms it is partially polarized, while the electron density is drawn to carbon, as to the more electronegative element.
It follows that in their molecules there are only C-C and C-H bonds. The former are formed by the overlap of two electron hybridized sp 3 orbitals of two carbon atoms, and the latter are formed by the overlap of the s orbital of hydrogen and the sp 3 orbital of carbon. The length of the C-C bond is 1.54 angstroms, and the length of the C-H bond is 1.09 angstroms.
Geometry of the methane molecule
Methane is the simplest alkane, consisting of just one carbon atom and four hydrogen atoms.
Due to the energy equality of its three 2p and one 2s orbitals, obtained as a result of sp 3 hybridization, all orbitals in space are located at the same angle to each other. It is equal to 109.47°. As a result of such a molecular structure, something like a triangular equilateral pyramid is formed in space.

Simple alkanes
The simplest alkane is methane, which consists of one carbon atom and four hydrogen atoms. Following methane in the series of alkanes, propane, ethane and butane are formed by three, two and four carbon atoms, respectively. Starting with five carbon atoms in the chain, the compounds are named according to IUPAC nomenclature.
A table with the formulas of alkanes and their names is given below:
When one hydrogen atom is lost from an alkane molecule, an active radical is formed, the ending of which changes from “an” to “yl”, for example, ethane C 2 H 6 - ethyl C 2 H 5. The structural formula of the alkane ethane is shown in the photo.

Nomenclature of organic compounds
The rules for determining the names of alkanes and compounds based on them are established by the international IUPAC nomenclature. For organic compounds the following rules apply:
- The name of a chemical compound is based on the name of its longest chain of carbon atoms.
- The numbering of carbon atoms should begin from the end, closer to which the chain begins to branch.
- If a compound contains two or more carbon chains of the same length, then the one that has the fewest radicals and has a simpler structure is chosen as the main one.
- If there are two or more identical groups of radicals in a molecule, then the corresponding prefixes are used in the name of the compound, which double, triple, and so on the names of these radicals. For example, instead of the expression “3-methyl-5-methyl”, “3,5-dimethyl” is used.
- All radicals are written in alphabetical order in the general name of the compound, and prefixes are not taken into account. The last radical is written together with the name of the chain itself.
- Numbers reflecting the numbers of radicals in the chain are separated from the names by a hyphen, and the numbers themselves are written separated by commas.
Following the rules of IUPAC nomenclature makes it easy to determine the molecular formula of an alkane, for example, 2,3-dimethylbutane has the following form.

Physical properties
The physical properties of alkanes largely depend on the length of the carbon chain forming the particular compound. The main properties are the following:
- The first four representatives, according to the general formula of alkanes, are in a gaseous state under normal conditions, that is, butane, methane, propane and ethane. As for pentane and hexane, they already exist in the form of liquids, and starting with seven carbon atoms, alkanes are solids.
- As the length of the carbon chain increases, the density of the compound increases, as well as its temperature of first-order phase transitions, that is, the melting and boiling temperatures.
- Since the polarity of the chemical bond in the formula of the substance of alkanes is insignificant, they do not dissolve in polar liquids, for example, in water.
- Accordingly, they can be used as good solvents for compounds such as non-polar fats, oils and waxes.
- A home gas stove uses a mixture of alkanes, rich in the third member of the chemical series - propane.
- When alkanes burn in oxygen, a large amount of energy is released in the form of heat, so these compounds are used as combustible fuel.
Chemical properties
Due to the presence of stable bonds in alkane molecules, their reactivity is low in comparison with other organic compounds.
Alkanes practically do not react with ionic and polar chemical compounds. They behave inertly in solutions of acids and bases. Alkanes react only with oxygen and halogens: in the first case we are talking about oxidation processes, in the second - about substitution processes. They also exhibit some chemical activity in reactions with transition metals.
In all these chemical reactions, the branching of the carbon chain of alkanes, that is, the presence of radical groups in them, plays an important role. The more there are, the more the ideal angle between bonds of 109.47° in the spatial structure of the molecule changes, which leads to the creation of stresses inside it and, as a result, increases the chemical activity of such a compound.
The reaction of simple alkanes with oxygen occurs according to the following scheme: C n H 2n+2 + (1.5n+0.5)O 2 → (n+1)H 2 O+ nCO 2 .
An example of a reaction with chlorine is shown in the photo below.

The danger of alkanes for nature and humans

Heptane, pentane and hexane are highly flammable liquids and are hazardous to both the environment and human health because they are toxic.
The table shows some representatives of a number of alkanes and their radicals.
|
Formula |
Name |
Radical name |
|||||||||||
|
CH3 methyl |
|||||||||||||
|
C3H7 cut |
|||||||||||||
|
C4H9 butyl |
|||||||||||||
|
isobutane |
isobutyl |
||||||||||||
|
isopentane |
isopentyl |
||||||||||||
|
neopentane |
neopentyl |
||||||||||||
|
The table shows that these hydrocarbons differ from each other in the number of groups - CH2 -. Such a series of similar structures, having similar chemical properties and differing from each other in the number of these groups is called a homologous series. And the substances that make it up are called homologues. Homologs - substances similar in structure and properties, but differing in composition by one or more homologous differences (- CH2 -)
Carbon chain - zigzag (if n ≥ 3) σ - bonds (free rotation around bonds) length (-C-C-) 0.154 nm binding energy (-C-C-) 348 kJ/mol All carbon atoms in alkane molecules are in a state of sp3 hybridization
the angle between the C-C bonds is 109°28", therefore the molecules of normal alkanes with a large number of carbon atoms have a zigzag structure (zigzag). The length of the C-C bond in saturated hydrocarbons is 0.154 nm (1 nm = 1 * 10-9 m). a) electronic and structural formulas; b) spatial structure
4. Isomerism- STRUCTURAL isomerism of the chain with C4 is characteristic One of these isomers ( n-butane) contains an unbranched carbon chain, and the other, isobutane, contains a branched one (isostructure). The carbon atoms in a branched chain differ in the type of connection with other carbon atoms. Thus, a carbon atom bonded to only one other carbon atom is called primary, with two other carbon atoms - secondary, with three - tertiary, with four - quaternary. With an increase in the number of carbon atoms in the molecules, the possibilities for chain branching increase, i.e. the number of isomers increases with the number of carbon atoms. Comparative characteristics of homologues and isomers
1. They have their own nomenclature radicals(hydrocarbon radicals)
| |||||||||||||
DEFINITION
Alkanes– saturated (aliphatic) hydrocarbons, the composition of which is expressed by the formula C n H 2 n +2.
Alkanes form a homologous series, each chemical compound of which differs in composition from the next and previous ones by the same number of carbon and hydrogen atoms - CH 2, and the substances included in the homologous series are called homologues. The homologous series of alkanes is presented in Table 1.
Table 1. Homologous series of alkanes.
In alkane molecules, primary (i.e. connected by one bond), secondary (i.e. connected by two bonds), tertiary (i.e. connected by three bonds) and quaternary (i.e. connected by four bonds) carbon atoms are distinguished.
C 1 H3 – C 2 H 2 – C 1 H 3 (1 – primary, 2 – secondary carbon atoms)
CH 3 –C 3 H(CH 3) – CH 3 (3-tertiary carbon atom)
CH 3 – C 4 (CH 3) 3 – CH 3 (4-quaternary carbon atom)
Alkanes are characterized by structural isomerism (carbon skeleton isomerism). Thus, pentane has the following isomers:
CH 3 -CH 2 -CH 2 -CH 2 -CH 3 (pentane)
CH 3 –CH(CH 3)-CH 2 -CH 3 (2-methylbutane)
CH 3 -C(CH 3) 2 -CH 3 (2,2 – dimethylpropane)
Alkanes, starting with heptane, are characterized by optical isomerism.
The carbon atoms in saturated hydrocarbons are in sp 3 hybridization. The angles between bonds in alkane molecules are 109.5.
Chemical properties of alkanes
Under normal conditions, alkanes are chemically inert - they do not react with either acids or alkalis. This is explained by the high strength of C-C and C-H bonds. Non-polar C-C and C-H bonds can only be cleaved homolytically under the influence of active free radicals. Therefore, alkanes enter into reactions that proceed by the radical substitution mechanism. In radical reactions, hydrogen atoms are first replaced at tertiary carbon atoms, then at secondary and primary carbon atoms.
Radical substitution reactions have a chain nature. The main stages: nucleation (initiation) of the chain (1) - occurs under the influence of UV radiation and leads to the formation of free radicals, chain growth (2) - occurs due to the abstraction of a hydrogen atom from the alkane molecule; chain termination (3) – occurs when two identical or different radicals collide.
X:X → 2X . (1)
R:H+X . → HX + R . (2)
R . + X:X → R:X + X . (2)
R . + R . → R:R (3)
R . +X . → R:X (3)
X . +X . → X:X (3)
Halogenation. When alkanes react with chlorine and bromine under the action of UV radiation or high temperature, a mixture of products from mono- to polyhalogen-substituted alkanes is formed:
CH 3 Cl +Cl 2 = CH 2 Cl 2 + HCl (dichloromethane)
CH 2 Cl 2 + Cl 2 = CHCl 3 + HCl (trichloromethane)
CHCl 3 +Cl 2 = CCl 4 + HCl (carbon tetrachloride)
Nitration (Konovalov reaction). When dilute nitric acid acts on alkanes at 140C and low pressure, a radical reaction occurs:
CH 3 -CH 3 +HNO 3 = CH 3 -CH 2 -NO 2 (nitroethane) + H 2 O
Sulfochlorination and sulfoxidation. Direct sulfonation of alkanes is difficult and is most often accompanied by oxidation, resulting in the formation of alkanesulfonyl chlorides:
R-H + SO 2 + Cl 2 → R-SO 3 Cl + HCl
The sulfonic oxidation reaction proceeds similarly, only in this case alkanesulfonic acids are formed:
R-H + SO 2 + ½ O 2 → R-SO 3 H
Cracking– radical cleavage of C-C bonds. Occurs when heated and in the presence of catalysts. When higher alkanes are cracked, alkenes are formed; when methane and ethane are cracked, acetylene is formed:
C 8 H 18 = C 4 H 10 (butane) + C 3 H 8 (propane)
2CH 4 = C 2 H 2 (acetylene) + 3H 2
Oxidation. The mild oxidation of methane with atmospheric oxygen can produce methanol, formic aldehyde or formic acid. In air, alkanes burn to carbon dioxide and water:
C n H 2 n +2 + (3n+1)/2 O 2 = nCO 2 + (n+1)H 2 O
Physical properties of alkanes
Under normal conditions, C 1 -C 4 are gases, C 5 -C 17 are liquids, and starting from C 18 are solids. Alkanes are practically insoluble in water, but are highly soluble in non-polar solvents, such as benzene. Thus, methane CH 4 (swamp, mine gas) is a colorless and odorless gas, highly soluble in ethanol, ether, hydrocarbons, but poorly soluble in water. Methane is used as a high-calorie fuel in natural gas, as a raw material for the production of hydrogen, acetylene, chloroform and other organic substances on an industrial scale.
Propane C 3 H 8 and butane C 4 H 10 are gases used in everyday life as bottled gases due to their easy liquefaction. Propane is used as a car fuel because it is more environmentally friendly than gasoline. Butane is the raw material for the production of 1,3-butadiene, which is used in the production of synthetic rubber.
Preparation of alkanes
Alkanes are obtained from natural sources - natural gas (80-90% - methane, 2-3% - ethane and other saturated hydrocarbons), coal, peat, wood, oil and rock wax.
There are laboratory and industrial methods for producing alkanes. In industry, alkanes are obtained from bituminous coal (1) or by the Fischer-Tropsch reaction (2):
nC + (n+1)H 2 = C n H 2 n +2 (1)
nCO + (2n+1)H 2 = C n H 2 n +2 + H 2 O (2)
Laboratory methods for producing alkanes include: hydrogenation of unsaturated hydrocarbons by heating and in the presence of catalysts (Ni, Pt, Pd) (1), the interaction of water with organometallic compounds (2), electrolysis of carboxylic acids (3), by decarboxylation reactions (4) and Wurtz (5) and in other ways.
R 1 -C≡C-R 2 (alkyne) → R 1 -CH = CH-R 2 (alkene) → R 1 -CH 2 – CH 2 -R 2 (alkane) (1)
R-Cl + Mg → R-Mg-Cl + H 2 O → R-H (alkane) + Mg(OH)Cl (2)
CH 3 COONa↔ CH 3 COO — + Na +
2CH 3 COO - → 2CO 2 + C 2 H 6 (ethane) (3)
CH 3 COONa + NaOH → CH 4 + Na 2 CO 3 (4)
R 1 -Cl +2Na +Cl-R 2 →2NaCl + R 1 -R 2 (5)
Examples of problem solving
EXAMPLE 1
| Exercise | Determine the mass of chlorine required for the first stage chlorination of 11.2 liters of methane. |
| Solution | Let us write the reaction equation for the first stage of methane chlorination (i.e., in the halogenation reaction, only one hydrogen atom is replaced, resulting in the formation of a monochlorine derivative): CH 4 + Cl 2 = CH 3 Cl + HCl (methane chloride) Let's find the amount of methane substance: v(CH 4) = V(CH 4)/V m v(CH 4) = 11.2/22.4 = 0.5 mol According to the reaction equation, the number of moles of chlorine and the number of moles of methane are equal to 1 mol, therefore, the practical number of moles of chlorine and methane will also be the same and will be equal to: v(Cl 2) = v(CH 4) = 0.5 mol Knowing the amount of chlorine substance, you can find its mass (which is what is posed in the problem question). The mass of chlorine is calculated as the product of the amount of chlorine substance and its molar mass (molecular mass of 1 mole of chlorine; molecular mass is calculated using the table of chemical elements by D.I. Mendeleev). The mass of chlorine will be equal to: m(Cl 2) = v(Cl 2)×M(Cl 2) m(Cl 2) = 0.5 × 71 = 35.5 g |
| Answer | The mass of chlorine is 35.5 g |